Efficacy of Acellular Dermal Matrix in Breast Augmentation for capsular contracture, review of an article
Introduction
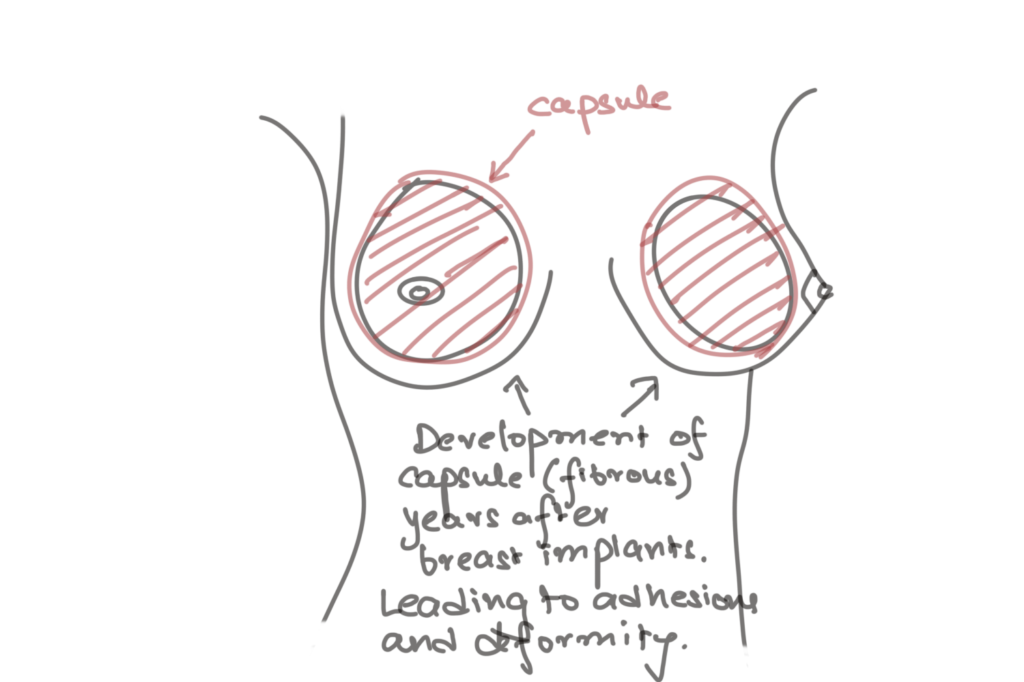
Capsular contracture is a major complication in breast augmentation procedures, causing discomfort, aesthetic distortion, and the need for revision surgeries. A recent systematic review and meta-analysis published in the Aesthetic Surgery Journal examined the effectiveness of different types of acellular dermal matrices (ADM) in reducing capsular contracture rates. This review explores the findings, clinical implications, and areas requiring further research.
Understanding Capsular Contracture
Capsular contracture occurs when a fibrous capsule surrounding a breast implant thickens and contracts, leading to firmness, pain, and aesthetic issues. The incidence ranges from 2% to 15% in augmentation patients, and treatment often necessitates surgical intervention [1]. Factors contributing to capsular contracture include implant texture, pocket placement, infection, hematoma, and shear forces [2].
Role of Acellular Dermal Matrix
ADMs are derived from human, porcine, or bovine dermis, processed to remove cellular components while preserving extracellular scaffolding. Their application in breast surgery aims to mitigate inflammatory responses and fibrosis, thereby reducing contracture risk. The systematic review analyzed nine studies, encompassing 481 breasts treated with ADM, and provided comparative insights into different ADM types [3].
Key Findings from the Systematic Review
- ADM Effectiveness in Reducing Contracture: The overall capsular contracture rate was significantly lower in patients treated with ADM. Strattice, AlloDerm, FlexHD, and DermaMatrix exhibited the lowest contracture rates, whereas NeoForm and SurgiMend had the highest (25%) [3].
- Statistical Significance in Efficacy Differences: Strattice had a 1.53% contracture rate and demonstrated a statistically significant reduction in risk (RR 0.14, 95% CI 0.06–0.31) when compared with conventional treatment [3].
- Complication Rates: While ADMs are effective, they carry risks. NeoForm and SurgiMend had the highest complication rates (50% and 12.5%, respectively), though overall complication rates remained low [3].
Implications for Clinical Practice
The findings suggest that Strattice and AlloDerm are promising options for reducing contracture risk, particularly in revision breast surgeries. However, ADM selection should consider potential complications, cost, and patient-specific factors. The study underscores the importance of personalized surgical planning and informed consent, as ADMs are currently used off-label for capsular contracture treatment in aesthetic surgery [4].
Limitations and Areas for Further Research
Despite promising results, the review highlights critical limitations:
- Lack of Randomized Controlled Trials (RCTs): Most included studies were retrospective, introducing potential biases [3].
- Small Sample Sizes: Limited data on certain ADM types restricts definitive conclusions on comparative efficacy [3].
- Variability in Surgical Techniques: Differences in implant texture, pocket irrigation, and post-operative care may confound results [3].
- Short Follow-Up Periods: Given that contracture can develop years after surgery, long-term studies are needed [3].
Future Research Directions
To strengthen evidence and guide clinical decisions, future studies should focus on:
- RCTs Comparing Different ADM Types: Prospective trials with standardized protocols will offer higher-quality evidence.
- Longitudinal Studies: Extended follow-up periods can assess long-term efficacy and recurrence rates.
- Cost-Benefit Analyses: Given the high cost of ADMs, studies should evaluate their economic viability relative to their clinical benefits.
- Exploring Alternative Biomaterials: Investigating synthetic or bioengineered matrices may provide more cost-effective solutions with comparable efficacy.
Key Takeaways
✅ ADM Use in Breast Revision Surgery
- ADMs are used for correcting implant malposition, capsular contracture, ptosis, stretch deformity, and implant rippling.
- A variety of ADMs are available, but their differences in clinical outcomes remain unclear.
✅ Summary of the Systematic Review Findings
- The meta-analysis included 9 studies (481 treated breasts), analyzing the effectiveness of Strattice, AlloDerm, FlexHD, SurgiMend, NeoForm, and DermaMatrix.
- Strattice (1.53% contracture rate) and AlloDerm, FlexHD, DermaMatrix (0% rate) were effective.
- SurgiMend and NeoForm showed the highest capsular contracture rates (25%).
- The statistical difference in ADM efficacy was significant (P < .001).
✅ Limitations of the Systematic Review
- The included studies were retrospective, not randomized controlled trials (RCTs).
- Small sample sizes for some ADM types limit definitive conclusions.
- Variability in study methodologies (different follow-up durations and surgical techniques).
- The authors acknowledge that differences in ADM efficacy remain inconclusive due to study limitations.
✅ Additional Evidence from Reconstruction Studies
- Other clinical studies suggest ADM use lowers capsular contracture risk in primary and revision reconstruction cases.
- In vitro and histological studies indicate ADMs reduce chronic inflammatory responses, thereby preventing contracture formation.
🛠 How Should Surgeons Choose ADMs?
- In the absence of definitive evidence, clinical experience with specific ADMs should guide selection.
- The authors personally prefer AlloDerm, Strattice, and Artia in both aesthetic and reconstructive breast surgery.
💡 Multifactorial Causes of Capsular Contracture
- Patient-, implant-, and surgery-related factors influence contracture formation.
- Contamination by biofilm-producing bacteria is a primary cause.
- Antibiotic irrigation protocols, including povidone-iodine solutions, can help mitigate biofilm formation.
🔬 Genetics and Surgical Technique Play a Role
- Some patients are genetically predisposed to an exaggerated immune response.
- Surgical precision in implant placement and handling ADM materials is crucial for reducing contracture risk.
Conclusions & Research Recommendations
📝 Does This Review Answer the Question of ADM Efficacy?
- The authors do not believe the meta-analysis provides a definitive answer on which ADM is superior.
- More randomized controlled trials (RCTs) are necessary for conclusive evidence.
📢 Future Research Should Focus on:
- RCTs comparing different ADMs under uniform conditions.
- Long-term follow-up studies to assess durability and contracture recurrence rates.
- Cost-benefit analysis of ADM use in revision and aesthetic procedures.
Final Thoughts
While the systematic review provides useful pooled data, the commentary emphasizes that clinical experience and best surgical practices remain critical until higher-quality research is available. Surgeons should take a multifactorial approach to capsular contracture prevention, incorporating ADM use alongside infection control and meticulous surgical techniques.
📌 Source: Aesthetic Surgery Journal, 2024.
The use of ADMs in breast augmentation revision surgeries significantly reduces capsular contracture rates, with Strattice and AlloDerm emerging as leading choices. However, further high-quality research is needed to refine best practices, optimize patient selection, and establish cost-effective strategies. Plastic surgeons must balance ADM benefits with potential complications and financial considerations while advancing surgical techniques to improve patient outcomes.
References
[1] Aesthetic Surgery Journal, “Efficacy of Acellular Dermal Matrix Type in Treatment of Capsular Contracture in Breast Augmentation,” 2024. DOI: 10.1093/asj/sjad265
[2] Adams WP. “Capsular Contracture: What is it? What Causes it? How Can it be Prevented and Managed?” Clin Plast Surg. 2009;36(1):119-126. DOI: 10.1016/j.cps.2008.08.007
[3] Maxwell GP, Gabriel A. “Use of Acellular Dermal Matrix in Revisionary Aesthetic Breast Surgery.” Aesthet Surg J. 2009;29(6):485-493. DOI: 10.1016/j.asj.2009.09.007
[4] Hidalgo DA, Weinstein AL. “Surgical Treatment for Capsular Contracture: A New Paradigm and Algorithm.” Plast Reconstr Surg. 2020;146(3):516-525. DOI: 10.1097/PRS.0000000000007079
